
Teaching basic chemistry and the periodic table can seem pretty daunting to some of us. Where do we start? How do we read and teach the periodic table of elements? What does everything on the periodic table even mean? So many questions!
Well, fret not! My unit study on the periodic table of elements and basic chemistry should make teaching this critical subject matter much easier. I take it one step at a time, and I break everything down to its basic elements. I promise that your kids will get the most out of this lesson and the activities included. I am also going to provide some tips and ideas on how to make this lesson more hands-on.
My unit study on the periodic table is designed for 6th through 8th-grade students. Feel free to include it or portions of it in your lesson plans.

The Periodic Table Unit Study covers the following topics:
- Student’s own periodic table for reference
- Atoms and atomic structure
- How to read the periodic table, including periods, groups, and families
- Elements and Compounds
- How to count atoms in compounds
- Metals, Metalloids, and Nonmetals
- Worksheets for each topic for practice
This post is written to supplement the unit study. The following sections provide information and key terms your students need to know.
Chemistry Basics
In 6th through 8th grades, students learn about the basics of chemistry, including atoms, elements, compounds, and the periodic table of elements. During this time, they will learn about the atomic structure and what makes up different atoms. They will learn about molecules, as well as the difference between an element and a compound. As they get into the periodic table, they will learn about specific elements and the properties of different elements. This unit study is designed to help improve your student’s understanding of each of these topics.
What is an Atom?
The atom is the basic building block of life. It is made up of three basic particles: protons, electrons, and neutrons. Protons have a positive charge and are located in the atom’s nucleus. Neutrons have no charge and are also located in the nucleus. Electrons have negative charges and are located in the electron cloud surrounding the nucleus. The electron cloud contains different energy levels where the electrons orbit the nucleus, and it is mostly empty space.

The opposite charges of the protons and electrons are what keep the electrons in their orbit around the atom’s nucleus. In a neutral atom, there is the same number of protons and electrons.
Atoms make up the elements, either as a single atom or multiple atoms. For instance, in their natural states, hydrogen (H2) and oxygen (O2) are made up of 2 atoms each, while carbon (C) and helium (He) are made up of 1 atom each.
When reading the periodic table of elements, the atomic number represents the number of protons and the number of electrons contained within the given element. The atomic mass is the combination of the number of protons and the number of neutrons in the nucleus. The mass of a proton or neutron is 1 atomic mass unit (amu).
Element vs. Compound
An element is a pure substance that cannot be broken down into simpler different substances. An element may contain more than one of the same atom, as we see above. Elements are represented by their chemical symbol found on the periodic table. For instance, C for carbon or He for helium.
Compounds are pure substances that consist of atoms of different elements that are bound together chemically. Water is the most common example of a compound. Compounds are represented by their chemical formula. The chemical formula for water is H2O.
In the unit study, I discuss the specific rules for identifying the elements in a compound, as well as the number of atoms of each element in the compound.
Periodic Table of the Elements
The periodic table is a chart of the chemical elements organized by atomic number, from hydrogen, the element with the lowest atomic number (1), to oganesson, the element with the highest atomic number (118). The atomic number of an element is the number of protons in the nucleus of an atom of that element. It is the number of protons that gives the element its identity, as no two elements have the same number of protons.
The elements in the periodic table are organized into groups, periods, and families.
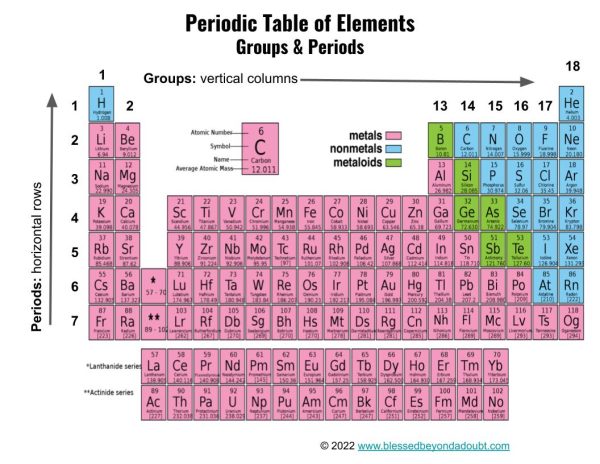
Groups are the vertical columns and tell us the number of valence electrons an atom has in the outer electron shell. For instance, the elements in Group 1 have 1 valence electron, and the elements in Group 18 have 8 valence electrons. The exception to this rule is helium in Group 18 which has 2 valence electrons.
Valence electrons are shared between atoms in chemical bonds and determine the element’s chemical properties, including reactivity. The elements in Group 18 are known as Noble Gases as they have full outer electron shells and are inert, meaning they do not react with other elements forming chemical bonds.
Periods are the horizontal rows and tell us the number of electron shells in an atom. There are 7 periods with Period 1 having one electron shell and Period 7 having 7 electron shells. A tip to remember the difference between Groups and Periods is “Sentences are read left to right and end in a Period“.
Example: Phosphorus is found in Group 15 and Period 3. It has 3 electron shells and 5 valence electrons in the outer shell.
Families of elements are classes of elements with similar chemical properties and include metals, metalloids, and nonmetals.

Metals consist of the elements on the left portion of the periodic table – Groups 1 through 11. Metals have specific characteristics, including shiny luster, they are solid, good conductors of heat and electricity, malleable, and ductile.
Metalloids are located along the “staircase” between metals and nonmetals, and they share characteristics with both metals and nonmetals. Metalloids are solid with a shiny luster, they are semiconductors, and most are brittle.
Nonmetals are located on the right side of the periodic table, and most nonmetals are gases, including halogens and noble gases. Nonmetals have a dull luster, they are poor conductors of heat and electricity, they are brittle, and they are neither malleable nor ductile.
Periodic Table Brain Dump
For those of you with 8th-grade students preparing for standardized testing in science, there are several videos on YouTube that show students how to use a “brain dump” on the periodic table. The brain dump provides all the information students will need to know for the test. I have found this method of reviewing the information about the periodic table very beneficial.
These videos are specific to the 8th-grade STAAR test given here in Texas, but the same technique can be used for any test on the periodic table.
Anchor Charts & Interactive Notebooks
You can even use various pages in this packet as anchor charts for your child’s interactive notebook and on the classroom wall. Interactive notebooks are a great way for students to keep notes and reflect on what they are learning. They are designed to engage and excite students. I recommend using a composition notebook, as not only are they inexpensive but your child does not have to worry about a mangled spiral binding. Also, the pages stay intact, unlike spiral notebooks
where pages tend to get ripped out easily.

I really love having my students create their own personal interactive notebooks. I let them design their own covers which they can put on the cover of the notebook. Another reason that I like composition notebooks. When designing their covers, the only requirements that I have are they must include their name, my name, class subject, and there cannot be any offensive content on the cover. I actually keep a roll of clear packing tape in my desk drawer to tape the students’ covers onto their notebooks so they don’t fall off.
There are many resources available online for how to design and utilize interactive notebooks.
The anchor charts and math fact pages I have included in my packet are great resources to have your students include in their notebooks.

Other educational resources on BBAD!
Please check out my many other educational resources available for free on my website.
- Phases of the Moon – teach your child about the 8 phases of the moon
- How Fast is Fast? – 6th-grade resource for teaching students how to calculate speed
- Math Facts Packet & Anchor Charts – math facts for kindergarten through 3rd grade
- If I was a Superhero Writing Prompts – interactive writing activity with coloring pages
- Ukraine History & Culture – have your child learn about Ukraine’s diverse history and culture; including activities, puzzles, and games
- Free Text Structure Anchor Chart – free anchor chart for interactive notebooks and classrooms
- Fact or Opinion Anchor Charts and Worksheets
- Growth Chart Mindset Posters for the Classroom
- And, many, many more!!!!!!!!
I created this FREE Periodic Table Unit Study with worksheets for my email subscribers. I hope you see an improvement in your child’s understanding of the periodic table and basic chemistry
Simply fill out the form below and you will instantly receive the PDF via email.
Please take note of printing tips:
Please copy and paste the link into a new browser tab to print.
Please download and save it on your hard drive immediately to avoid the link becoming expired
Thank you for visiting Blessed Beyond a Doubt and pinning this activity for others to enjoy!!!










Leave A Comment